Alkalinity
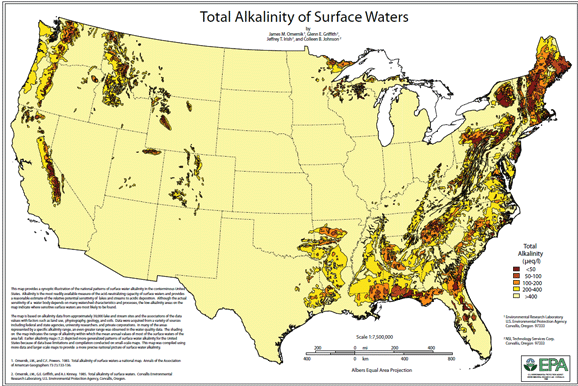 The U.S. Environmental Protection Agency produced a map illustrating the regional patterns of mean annual alkalinity of surface water in the conterminous United States. The map provides a qualitative graphic overview to the sensitivity of surface waters to acidification. The map is based on data from approximately 2,500 streams and lakes and apparent spatial correlations between these data and macrowatershed characteristics, especially land use.
The U.S. Environmental Protection Agency produced a map illustrating the regional patterns of mean annual alkalinity of surface water in the conterminous United States. The map provides a qualitative graphic overview to the sensitivity of surface waters to acidification. The map is based on data from approximately 2,500 streams and lakes and apparent spatial correlations between these data and macrowatershed characteristics, especially land use.
Alkalinity is a measure of the capacity of water to neutralize acids (see pH description). Alkaline compounds in the water such as bicarbonates (baking soda is one type), carbonates, and hydroxides remove H+ ions and lower the acidity of the water (which means increased pH). They usually do this by combining with the H+ ions to make new compounds. Without this acid-neutralizing capacity, any acid added to a stream would cause an immediate change in the pH. Measuring alkalinity is important in determining a stream’s ability to neutralize acidic pollution from rainfall or wastewater. It’s one of the best measures of the sensitivity of the stream to acid inputs.
Total alkalinity is measured by measuring the amount of acid (e.g., sulfuric acid) needed to bring the sample to a pH of 4.2. At this pH all the alkaline compounds in the sample are “used up.” The result is reported as milligrams per liter of calcium carbonate (mg/L CaCO3).
Source: http://water.usgs.gov/owq/hardness-alkalinity.html
Alkalinity is important because it protects or buffers against rapid pH changes.
Alkalinity is a measure of the buffering capacity of water – its ability to resist sudden changes in pH. pH is a measure of how acidic or basic water is.
pH Description:
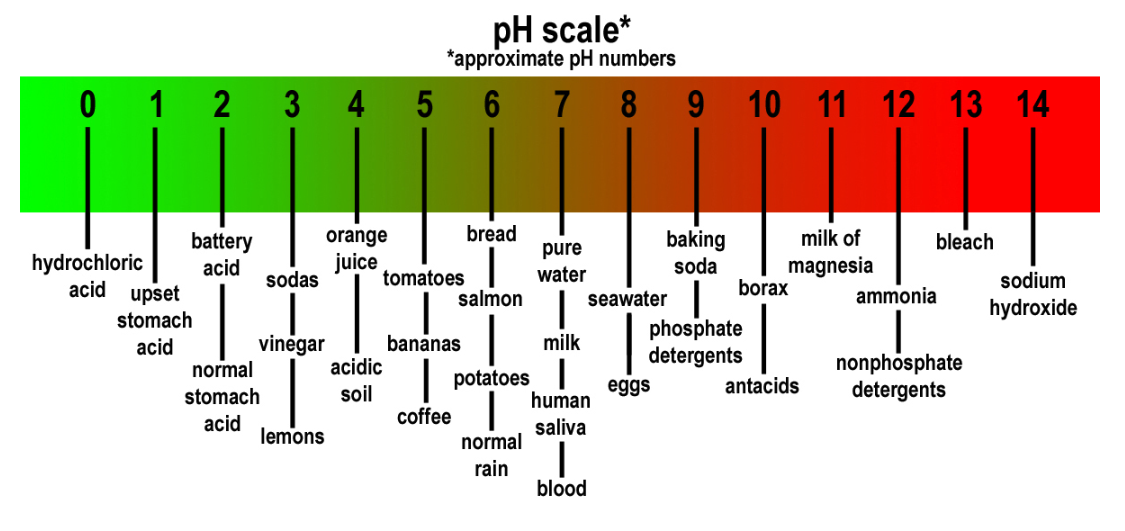
What is PH?
Acidic and basic are two extremes that describe chemicals, just like hot and cold are two extremes that describe temperature. Mixing acids and bases can cancel out their extreme effects; much like mixing hot and cold water can even out the water temperature. A substance that is neither acidic nor basic is neutral.
The pH scale measures how acidic or basic a substance is. It ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic, and a pH greater than 7 is basic. Each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times (10 times 10) more acidic than a pH of 6. The same holds true for pH values above 7, each of which is ten times more alkaline—another way to say basic—than the next lower whole value. For example, a pH of 10 is ten times more alkaline than a pH of 9.
Pure water is neutral, with a pH of 7.0. When chemicals are mixed with water, the mixture can become either acidic or basic. Vinegar and lemon juice are acidic substances, while laundry detergents and ammonia are basic.
Chemicals that are very basic or very acidic are called “reactive.” These chemicals can cause severe burns. Automobile battery acid is an acidic chemical that is reactive. Automobile batteries contain a stronger form of some of the same acid that is in acid rain. Household drain cleaners often contain lye, a very alkaline chemical that is reactive.
Note: Water that is absolutely pure will have a pH of 7. It is 7 because of the self-ionization of water. Water always has some H+ and some OH- ions and they are exactly equal in concentration at 10 to the minus 7 at the neutral pH of 7.
The reason water treated with reverse osmosis has an acidic pH is due to the fact that the minerals are completely removed and pure water will absorb CO2 from the atmosphere,forming the weak acid H2CO3, carbonic acid. This is why water that is saturated in CO2 will have a pH of 5.5, due to slight amount of acidity it gets from the H2CO3.The RO does not, by itself, remove gaseous CO2. So “Water after Reverse Osmosis” is going to be slightly different than water that is at theoretical maximum purity.The absence of the minerals lowers the pH balance of the water. Pure water, unless it is stored in a manner that excludes CO2, will become exactly like RO water if exposed to the air.

